Transform or Just Change? Exploring the Magic of Physical and Chemical Reactions
Physical and Chemical Changes Experiment For Class 9
Curiosity Questions
-
Why does iron turn reddish-brown when placed in a copper sulphate solution?
-
Can you reverse the burning of a magnesium ribbon?
-
What exactly makes a change physical or chemical?
STEM Challenge Introduction
In this hands-on STEM experiment, you’ll investigate everyday reactions and identify whether they are physical or chemical changes. Understanding this difference helps us comprehend the world better from cooking food to rusting metal to mixing chemicals in labs. You will carry out five mini-reactions that represent both types of changes. Get ready to think like a scientist, observe carefully, and classify the changes you see!
Materials Required
-
Iron nail or iron strip (cleaned with sandpaper).
-
Copper sulphate solution (100 ml).
-
Small test tube or beaker.
-
Magnesium ribbon (2-3 cm).
-
Spirit lamp or candle and tongs.
-
Zinc granules (2 g).
-
Dilute sulphuric acid (H₂SO₄, 10 ml, 1:10 dilution).
-
Blue copper sulphate crystals (2 g).
-
Bunsen burner or candle.
-
Sodium sulphate solution (10 ml).
-
Barium chloride solution (10 ml).
-
Dropper, test tubes, glass rod.
-
Safety goggles and gloves.
Eco-Friendly Alternatives:
Use household vinegar in place of acid for a milder reaction (though less dramatic). Use old keys or nails for iron.
Safety Precautions
-
Always wear gloves and goggles when handling acids or reactive metals.
-
Perform all heating steps under adult supervision.
-
Do not touch or taste any chemical.
-
Dispose of chemicals responsibly after completion.
Step-by-Step Process
1. Iron + Copper Sulphate Solution
-
Place an iron nail into a test tube containing copper sulphate solution.
-
Leave it for 20 minutes.
2. Burning Magnesium Ribbon
-
Hold a magnesium ribbon with tongs.
-
Ignite it over a flame. Observe the bright white flame.
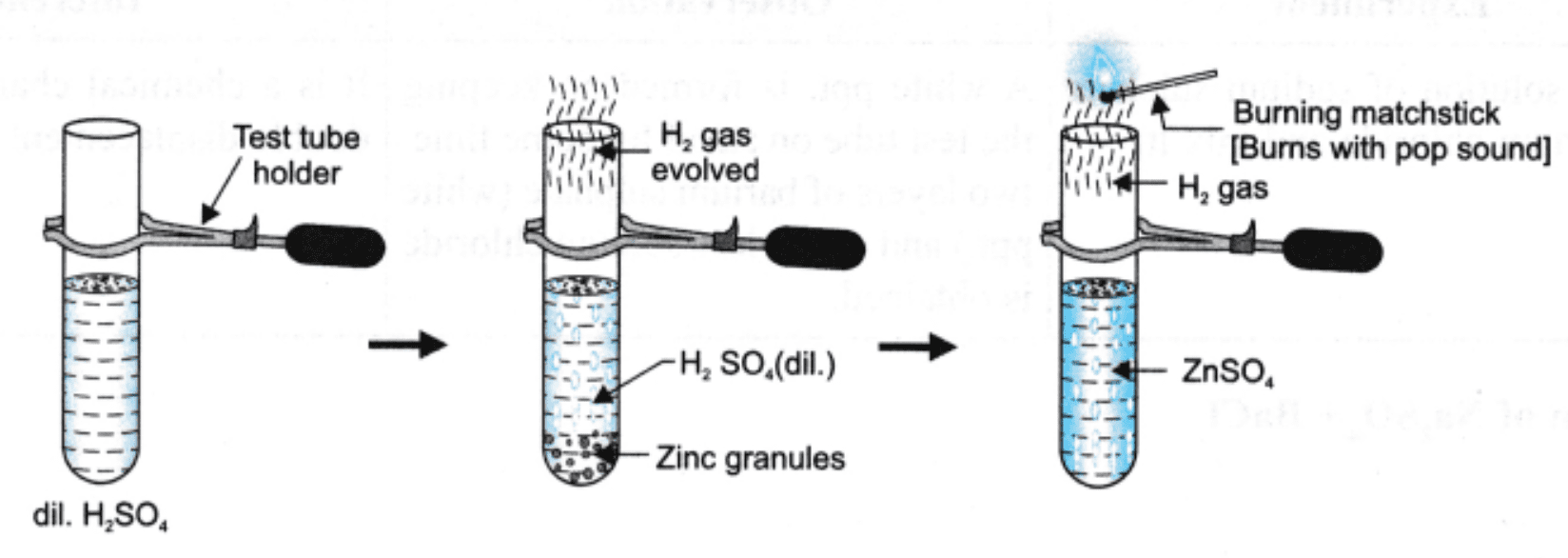
3. Zinc + Dilute Sulphuric Acid
-
Add a few zinc granules to a test tube.
-
Add dilute sulphuric acid slowly. Observe effervescence.
4. Heating Copper Sulphate Crystals
-
Place blue copper sulphate crystals in a dry test tube.
-
Heat gently. Observe the color change.
-
After cooling, add a drop of water to the white powder.
5. Sodium Sulphate + Barium Chloride Solution
-
Take 5 ml of sodium sulphate in a test tube.
-
Add 5 ml of barium chloride. Mix with a glass rod.
Expected Outcome
-
Iron + Copper Sulphate: A reddish-brown coating appears on the iron.
-
Burning Magnesium: Bright white light and a white powder (magnesium oxide).
-
Zinc + Dil. H₂SO₄: Bubbling or fizzing; hydrogen gas released.
-
Heating Copper Sulphate: Blue color disappears on heating, returns after water is added.
-
Sodium Sulphate + Barium Chloride: A white precipitate forms instantly.
Observation
-
What visible signs helped you identify a chemical reaction?
-
Which change do you think is reversible? Why?
-
Was any gas produced or precipitate formed?
STEM Concepts Behind It
-
Chemical Changes involve the formation of new substances and are usually irreversible. Signs include color change, gas evolution, precipitate formation, and energy change.
-
Physical Changes are usually reversible, and no new substance is formed.
-
Examples: Heating copper sulphate is physical (reversible by adding water). Burning magnesium is chemical (forms new substance magnesium oxide).
Concept Elaboration
This experiment links multiple branches of STEM:
-
Science: Understanding chemical properties and reaction types.
-
Technology: Using lab equipment safely.
-
Engineering: Designing safe experiments with proper observation.
-
Mathematics: Measuring liquids and comparing reaction times or quantities.
Recognizing whether a process is reversible helps scientists make predictions and engineers to choose suitable materials.
Real-Life Applications
-
Rusting of iron (a chemical change) affects construction, requiring coatings like paint or galvanization.
-
Precipitation reactions are used in wastewater treatment.
-
Gas-producing reactions like zinc and acid are used in portable hydrogen generation for lab-scale experiments.
-
Heating crystals and observing changes is used in pharmaceutical drying processes.
Your STEM Challenge
Can you design an experiment using kitchen materials to show a chemical change? (Hint: Think lemon juice + baking soda.)
Make a table comparing at least three physical and three chemical changes from your home.
Think Further & Explore More
-
Can you identify if boiling of water or melting of ice are physical or chemical changes?
-
Why do some changes produce heat or light while others don’t?
-
Explore further: Try the vinegar and eggshell reaction or baking soda and vinegar balloon experiment.
Refer This Video : Physical and Chemical Changes
Explore More : Sound Experiment For Class 9