Whats in Your Kitchen? Exploring the Power of Acids, Bases & Salts
Acids Bases and Salts Lesson Plan For Class 10
Hook – Whats Happening in the Kitchen?
Imagine this: You bite into a tangy orange, and your mouth tingles. Later, you sip soap water by mistake and instantly spit it out it tastes bitter and soapy. Then, your mom adds baking soda to the batter, and the cake magically rises in the oven.
What do all these have in common? They are all clues to something happening at a deeper chemical level something thats happening all around you.
Learning Objectives
By the end of this lesson, you will be able to:
-
Define acids, bases, and salts in simple terms.
-
Use indicators to identify them.
-
Understand how neutralization works.
-
Explain the importance of pH in real life.
-
Know how common substances like washing soda or plaster of Paris are made and used.
Key Vocabulary
-
Acid: A substance that releases H⁺ (hydrogen) ions in water. It tastes sour.
-
Base: A substance that releases OH⁻ (hydroxide) ions in water. It tastes bitter and feels slippery.
-
Salt: A compound formed when an acid reacts with a base.
-
Indicator: A substance that changes colour in acids or bases.
-
pH: A number scale (0-14) showing how acidic or basic a substance is.
-
Neutralization: A reaction between an acid and a base to form salt and water.
Curiosity Questions
-
Why do lemon and vinegar taste sour but soap tastes bitter?
-
Why do doctors check acidity in your stomach?
-
Can toothpaste neutralize acid? Why do they say it “protects your enamel”?
Topic Introduction
Acids, bases, and salts are not just part of a textbook they are part of your kitchen, your body, and even your garden soil. In this chapter, we are going to dive into what makes substances acidic or basic, how we can detect them, and what happens when they mix. Understanding these chemicals is essential because they play a huge role in health, environment, and industry.
Analogies to Understand Concepts
-
Acids and Bases Are Like Opposites on a See saw: Acids tip to one side with H⁺ ions, bases to the other with OH⁻. Neutralization balances them.
-
Indicators Are Mood Rings: Just like mood rings change color with temperature, indicators change color with acids or bases.
-
pH Scale Is a Thermometer for Chemicals: Just like temperature tells you hot or cold, pH tells how acidic or basic something is.
Core Concept Explanation
Acids and Bases
-
Acid: Any substance that releases hydrogen ions (H⁺) when dissolved in water. Example: Hydrochloric acid (HCl).
-
Base: Any substance that releases hydroxide ions (OH⁻) in water. Example: Sodium hydroxide (NaOH).
Indicators
-
Litmus (blue/red), methyl orange, and phenolphthalein are common indicators.
-
Litmus turns red in acids, blue in bases.
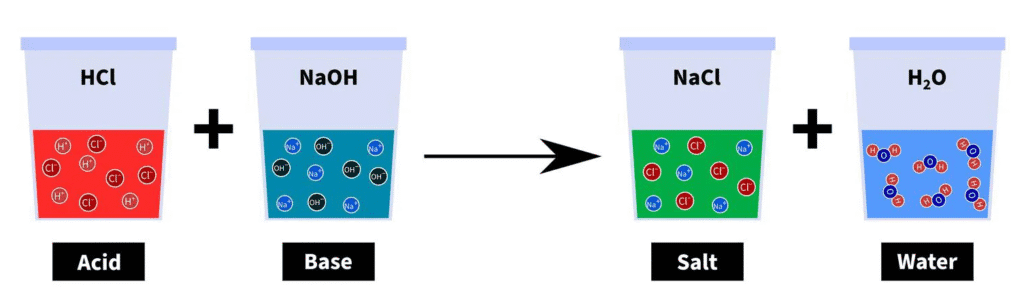
Neutralization Reaction
Acid + Base → Salt + Water
Example: HCl + NaOH → NaCl + H₂O
pH Scale
-
Scale ranges from 0 to 14
-
0-6: Acidic
-
7: Neutral
-
8-14: Basic
Preparation of Salts
-
Sodium hydroxide: By electrolysis of saltwater.
-
Bleaching powder: Reacting chlorine with dry slaked lime.
-
Baking soda: From sodium carbonate and CO₂.
-
Washing soda: From baking soda upon heating.
-
Plaster of Paris: From gypsum heated at 373K.
Scale or Context
If your stomach’s pH drops below 2, you will feel acidity. Shampoo has a pH around 5-7. Swimming pools are kept around pH 7.2-7.8 to prevent skin irritation. These small changes have big effects on daily life.
DIY Hands On Activity: Test Your Kitchen Chemistry
Purpose: Identify acids and bases using natural indicators.
Materials:
-
Red cabbage or turmeric powder
-
Lemon juice, vinegar, baking soda, soap water
-
Water, bowls, dropper, white plate
Safety: Do not ingest anything. Wash hands after the activity.
Steps:
-
Make red cabbage extract by boiling chopped cabbage in water. Cool and filter.
-
Place drops of lemon juice, vinegar, soap water, and baking soda on a plate.
-
Add cabbage extract and observe colour change.
-
Repeat using turmeric mixed in water.
Concept Connection & Deep Explanation
What did we observe?
-
Lemon juice turned cabbage extract pink (acid).
-
Soap water turned it green/blue (base).
-
Turmeric turned red with base (alkaline).
Why did this happen?
Indicators react with H⁺ and OH⁻ ions to show different colours.
What does it show?
We can test substances using natural indicators to know if they are acidic or basic.
Observation / Exploration
Try testing pH of shampoo, toothpaste, cola, or soap using red cabbage indicator. Record your observations and see how products are designed for safe pH.
Elaboration Activity
Role play: Act out a “pH rescue squad” where acids and bases are characters who fight, and neutralization is the peace treaty. Add baking soda as a doctor who cures excess acidity.
Real-life Applications
-
Toothpaste: Mildly basic to neutralize acids from food and protect teeth.
-
Soil Testing: Farmers use pH tests to balance soil for healthy crops.
-
Medicine: Antacids like milk of magnesia neutralize stomach acid.
Quick Quiz
-
What ion do acids release in water?
a) OH⁻ b) H⁺ c) Na⁺ -
Which substance is a base?
a) Vinegar b) Lemon juice c) Baking soda -
What is the pH of a neutral solution?
Think Pair Share
What if the water in your town became acidic due to pollution? How could this affect human health, farming, and aquatic life?
Main Recap
-
Acids release H⁺, bases release OH⁻.
-
Indicators show colour change to test pH.
-
pH tells how acidic or basic something is.
-
Neutralization makes salt and water.
-
Everyday items like toothpaste and baking soda are based on this science.
Creative Challenge
Design a comic strip titled “The Battle of the Ions” where acid and base superheroes use their powers, and the pH scale determines their strength. Include neutral as the peacemaker.
More to Explore
-
Look up “Why is stomach acid important?”
-
Visit PhET interactive simulations on pH scale.
-
Watch a chemistry magic trick using red cabbage indicator.
Student Self-Evaluation
☐ I can explain what acids and bases are.
☐ I know how to test substances using indicators.
☐ I understand why pH is important.
☐ I enjoyed the activity and learned something new.
Reflection
What was your favourite part of this lesson? What surprised you the most? Write 2-3 sentences about what you learned and how you might use this knowledge in real life.
Digital Learning Enhancements
YouTube Animation Link: Acid Bases and Salts
Interactive PhET Simulation: Acid Base Solutions