Current in Action: Uncover the Hidden Chemistry of Electricity and Electroplating
Chemical Effects of Electric Current Experiment For Class 8
Curiosity Questions
-
Can electricity cause a chemical change in a liquid?
-
Why are gold and silver coatings found on cheaper metals in jewelry?
-
How can we tell if a liquid is a good conductor of electricity?
STEM Challenge Introduction
In this exciting STEM experiment, you’ll explore how electricity can do more than just power devices it can change substances chemically. You will test the conductivity of various liquids and learn how electroplating is done using a simple setup. Through this activity, you’ll discover how electric current can break molecules apart and even help coat one metal onto another like copper on iron. It’s not just science; it’s a peek into how industries work.
Materials Required
Testing Conductivity:
-
3–4 test liquids (e.g., lemon juice, salt water, tap water, distilled water, vinegar).
-
A 9V battery.
-
Battery holder with wires.
-
Small bulb (torch bulb or LED).
-
2 iron nails.
-
Wires with alligator clips.
-
Cardboard or plastic stand.
-
Beaker or plastic cup.
Electroplating Activity:
-
Copper sulphate solution (100 ml).
-
Iron nail or key.
-
Copper plate or old copper coin.
-
9V battery.
-
Battery holder and wires.
-
Beaker.
-
Sandpaper.
Eco-Friendly Alternatives:
-
Use old copper wires or coins for electrodes.
-
Use vinegar and salt mixture if copper sulphate is not available.
Safety Precautions
-
Always handle chemicals like copper sulphate with care. Avoid touching it directly.
-
Do not connect the battery for too long it may overheat.
-
Adult supervision is recommended while setting up the circuit and dealing with liquids.
-
Wash hands after the experiment.
Step-by-Step Process
Testing Conductivity of Liquids
-
Connect wires from the battery to two iron nails using alligator clips.
-
Mount the nails on a cardboard so that their tips are dipped into the liquid but don’t touch each other.
-
Connect a bulb between the wires.
-
Dip the nails into each test liquid one by one and observe if the bulb glows.
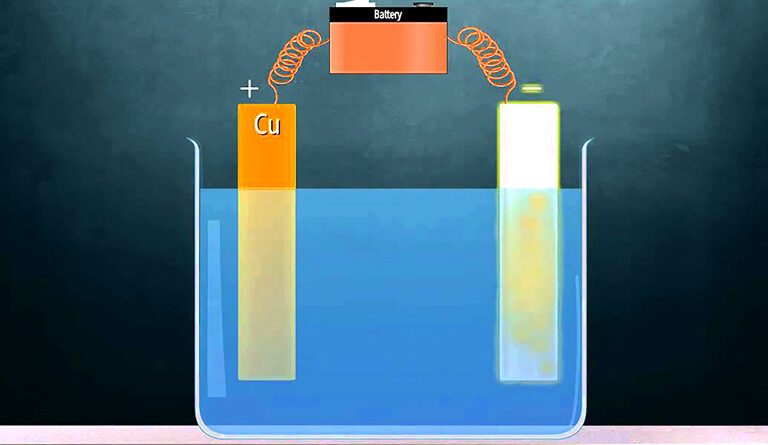
Electroplating
-
Clean the iron nail or key and copper plate using sandpaper.
-
Connect the iron nail to the negative terminal of the battery (cathode).
-
Connect the copper plate to the positive terminal (anode).
-
Pour copper sulphate solution into the beaker.
-
Dip both electrodes into the solution and keep them undisturbed for 20-30 minutes.
-
After some time, remove the iron nail and observe the changes.
Expected Outcome
-
Some liquids like lemon juice, salt water, and vinegar will allow the bulb to glow, indicating they conduct electricity. Distilled water will not.
-
After electroplating, a reddish-brown layer of copper will be seen on the iron nail, indicating a chemical change caused by electric current.
Observation
-
Which liquids made the bulb glow?
-
What changes did you observe on the iron nail after electroplating?
-
Was there any gas or color change noticed in the liquid?
STEM Concepts Behind It
-
Science: Electricity can break down molecules in liquids, producing chemical changes like metal deposition or gas formation.
-
Technology: Electroplating is used in many industrial processes.
-
Engineering: Building circuits and using safe methods to transfer metals onto surfaces.
-
Math: Measuring quantities of liquids and duration of current flow.
Concept Elaboration
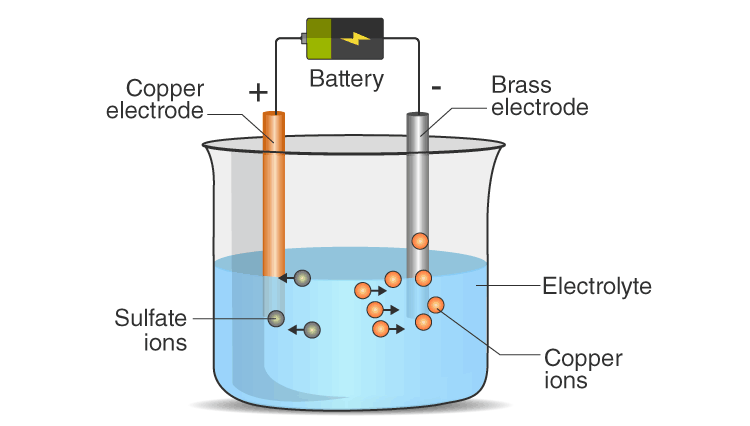
When electric current passes through a conducting solution, it causes electrolysis breaking down the solution into its components. The positive ion (Cu²⁺) from copper sulphate moves towards the negative terminal and gets deposited as solid copper on the iron nail. This is a chemical effect of electric current, unlike heating or lighting effects.
Real-Life Applications
-
Electroplating is used to coat cheaper metals with silver, gold, or chromium in industries like jewelry, kitchenware, and automobile parts.
-
Battery manufacturing involves the chemical effect of electric current to create chargeable materials.
-
Purification of metals in metallurgical industries uses similar principles to refine copper or aluminum.
Your STEM Challenge
Try electroplating an old key using a vinegar and salt solution with a copper coin. Record your observations and compare the thickness of the coating after 30 minutes and 1 hour.
Bonus: Can you try it with graphite electrodes (from pencil leads) instead of metal?
Think Further & Explore More
-
What happens if you reverse the battery connections in electroplating?
-
Can fruit juices conduct electricity? Why or why not?
-
Explore more: Investigate the electrolysis of water to produce hydrogen and oxygen using a simple setup.
Watch this – Chemical Effects of Electric Current Animation Video
Also Explore Here – Force and Pressure Experiment For Class 8