Ignite the Science: Exploring the Hidden Secrets of Combustion and Flame
Combustion and Flame Lesson Plan for Class 8
Hook
Imagine this: You’re camping at night in the forest. Someone strikes a match, and suddenly, warmth and light burst into existence. What really happened in that split second? Why does fire behave that way?
Learning Objectives
By the end of this lesson, students will be able to:
-
Understand what combustion is and what conditions are required for it.
-
Identify and differentiate between types of combustion.
-
Describe the structure of a flame.
-
Understand fuel efficiency and calorific value.
-
Explain fire control and the use of fire extinguishers.
Key Vocabulary
-
Combustion.
-
Ignition temperature.
-
Inflammable substances.
-
Rapid combustion.
-
Spontaneous combustion.
-
Explosion.
-
Flame.
-
Luminous and non-luminous zones.
-
Calorific value.
-
Fire extinguisher.
Curiosity Questions
-
Why do candles melt and shine at the same time?
-
How do firecrackers explode with such energy and color?
-
Why do firemen use foam or CO₂ to put out fires?
Topic Introduction
Combustion is a chemical process where a substance reacts with oxygen to produce heat and light. We see it when something burns like wood, cooking gas, or candles. This process is not only a source of energy but also a part of daily life and safety.
Analogies to Build Understanding
-
Combustion is like cooking popcorn You need the right temperature (ignition), a base (oil), and popcorn (fuel). If one is missing, nothing pops.
-
Flame zones are like a layered cake Each part has its own texture (temperature, color, use), and cutting it reveals different “layers” of activity.
Core Concept Explanation
What is Combustion?
Combustion is the process of burning a substance in the presence of oxygen to release heat and sometimes light. The minimum temperature required to start this process is called ignition temperature. Substances that catch fire easily at low temperatures are called inflammable substances (like petrol, alcohol, LPG).
Conditions Needed for Combustion
-
Presence of a combustible material (fuel).
-
Oxygen (air).
-
Heat (to reach ignition temperature).
Types of Combustion
-
Rapid Combustion – e.g., LPG burns instantly on igniting.
-
Spontaneous Combustion – e.g., coal dust catching fire without a flame.
-
Explosion – e.g., firecrackers, where heat and gas are suddenly released.
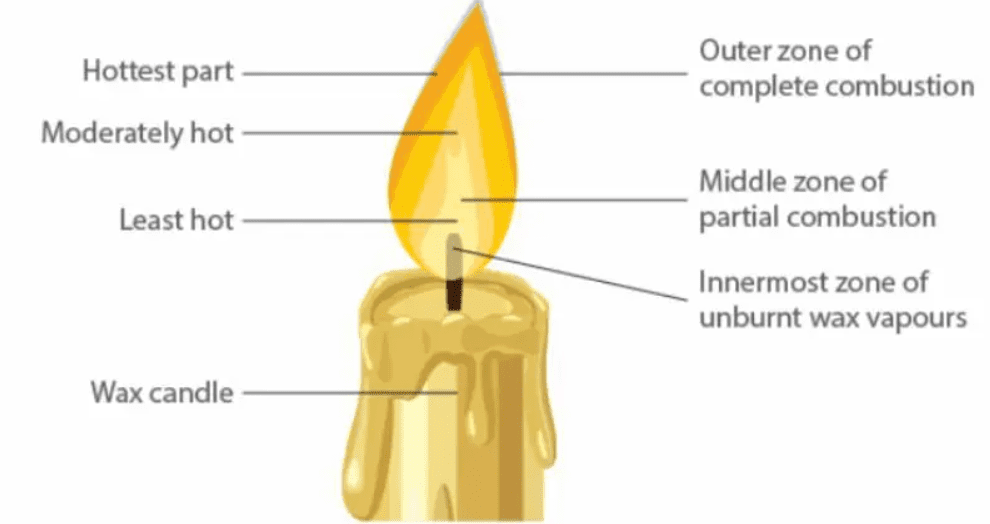
Structure of a Flame
A flame has three zones:
-
Outer zone (blue, hottest, complete combustion).
-
Middle zone (yellow, moderately hot, partial combustion).
-
Inner zone (black, least hot, unburnt fuel vapors).
Fuel and Efficiency
A good fuel has high calorific value (amount of heat per gram), burns cleanly, and is easy to transport. LPG, biogas, and CNG are examples of efficient fuels.
Fire Control & Fire Extinguishers
Fire can be controlled by:
-
Removing fuel.
-
Cutting off air (oxygen).
-
Lowering temperature.
Common extinguishers:
-
Water (for wood, paper).
-
Foam and CO₂ (for electrical or oil fires).
Scale or Context
-
A car engine runs because of controlled combustion.
-
A rocket launch is a result of a massive explosion (rapid combustion).
-
Your kitchen stove is an example of steady flame combustion.
DIY Hands-on Activity: Flame Zone Observation
Brief Overview:
Students will observe different zones of a candle flame and understand their properties.
Materials Needed:
-
Candle.
-
Matchstick.
-
Steel spoon or metal strip.
-
Notebook & pencil.
Safety Precautions:
-
Adult supervision.
-
Tie back loose hair.
-
Use flame-resistant surface.
Instructions:
-
Light a candle and observe the flame.
-
Hold a metal spoon over the flame for a few seconds (not too close).
-
Observe soot deposition and note the color of different flame zones.
-
Try to identify hot zones by feeling the heat (carefully).
Observation/Exploration Task
Ask students to observe the color of the flame in a gas stove and compare it with a candle flame. Which is hotter and why?
Elaboration Activity
Group Challenge:
Divide students into teams. Ask each team to act as Fire Safety Engineers and design a poster that educates people on:
-
How to prevent house fires.
-
How to use fire extinguishers.
-
Emergency contact numbers and do’s and don’ts during fire.
Explanation & Recap
Combustion powers much of the modern world from your stove to engines. It needs fuel, heat, and oxygen. Understanding its types, the flame structure, and fire control helps us use it safely and efficiently.
Real-Life Applications
-
Gas burners and stoves use complete combustion to provide heat for cooking.
-
Automobiles use controlled combustion to power engines.
Quick Quiz (3 Questions)
-
What are the three essential requirements for combustion?
-
Which part of the flame is the hottest?
-
Name one example of spontaneous combustion.
Think-Pair-Share
“Why is water not used to extinguish an oil fire or electrical fire? Discuss with your partner.”
Main Recap
-
Combustion = Burning + Oxygen + Heat.
-
Types = Rapid, Spontaneous, Explosion.
-
Flame Zones = Outer (hottest), Middle, Inner.
-
Fuels = Must have high calorific value.
-
Fire Control = Remove one of the three elements.
Creative Challenge
Design a fire extinguisher for a jungle where there’s no water available. What material would you use? How will it stop fire?
More to Explore
-
Learn about Green Fuels like ethanol and hydrogen.
-
Explore how space shuttles use combustion to launch.
-
Investigate how fireworks create colors (hint: metal salts!).
Student Self-Evaluation
-
I can explain what combustion is: Yes / No
-
I know how to control fire safely: Yes / No
-
I can describe flame zones and fuel efficiency: Yes / No
Reflection
What was the most surprising thing you learned today? Which activity did you enjoy the most?
Digital Learning Enhancements
YouTube Animation: Combustion and Flame Video
Also Click Here : Friction Lesson Plan For Class 8