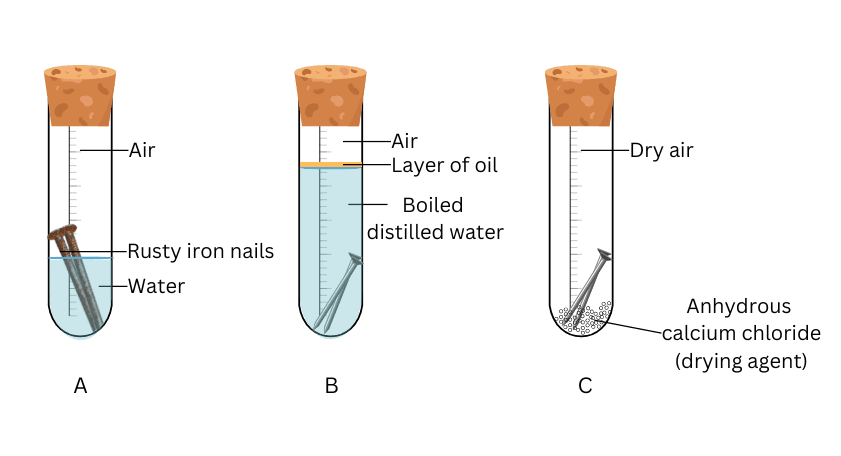Metal Wars: Reactivity, Rust, and Real-World Wonders
Metals and Non Metals Experiment For Class 10
Curiosity Questions
-
Why do some metals react violently with water while others remain unaffected?
-
What happens when a metal strip is dipped into a solution of another metal’s salt?
-
Why do bridges and iron gates need to be painted regularly?
STEM Challenge Introduction
Have you ever seen an iron gate rust or wondered why certain metals sparkle while others dull over time? In this STEM challenge, you’ll explore the dynamic world of metals and non-metals by testing their reactivity with water and acids, observing displacement reactions, and exploring the corrosion process. You’ll also experiment with the physical properties of materials like malleability, ductility, and electrical conductivity to understand why metals play a crucial role in engineering, electronics, and everyday life.
Materials Required
Reactivity and Displacement Reactions:
-
Small samples of metals: sodium (Na), calcium (Ca), aluminum (Al), iron (Fe), and zinc (Zn) (approx. 1g each).
-
Dilute hydrochloric acid (HCl), 50 mL.
-
Water in a beaker (100 mL).
-
Copper sulfate (CuSO₄) solution, 50 mL.
-
Test tubes, test tube rack.
-
Sandpaper (to clean metal surfaces).
-
Dropper or pipette.
-
Tongs or forceps.
Property Testing:
-
Thin aluminum or copper wires (for ductility).
-
Metal sheets or foils (for malleability test).
-
Electrical circuit setup with bulb and battery (for conductivity).
-
Piece of graphite (as non-metal conductor).
-
Iron nail and water for rusting demonstration.
Eco-friendly Tips:
-
Reuse metal samples where safe.
-
Use minimal quantities of chemicals.
-
Use plastic-free tools like glass droppers and metal stands.
Safety Precautions
-
Always wear gloves and safety goggles when handling acids or reactive metals.
-
Conduct the sodium and calcium experiments under adult supervision or skip these if not available in a safe setting.
-
Wash hands after handling chemicals.
-
Do not inhale fumes directly.
-
Work in a well-ventilated area or under a fume hood.
Step-by-Step Process
Reactivity with Water and Acids
-
Place small pieces of Na, Ca, Al, and Fe separately in test tubes with water.
-
Observe and note which metals bubble, float, or remain unchanged.
-
Repeat the process with dilute HCl instead of water.
-
Record the speed and intensity of each reaction.
Displacement Reaction
-
Clean a zinc strip using sandpaper.
-
Place it in a test tube containing CuSO₄ solution.
-
Leave it undisturbed for 15-30 minutes and observe color change and surface deposition.
Testing Physical Properties
-
Bend metal sheets gently to test malleability.
-
Stretch or twist thin wires to observe ductility.
-
Complete a simple circuit using different materials (metal wires, graphite) and test for conductivity.
-
Leave an iron nail dipped in water for a few days and observe rust formation.
Expected Outcome
-
Sodium and calcium will react vigorously with water, producing hydrogen gas and heat.
-
Zinc will displace copper from CuSO₄, forming a reddish-brown deposit and turning the solution pale.
-
Malleable metals will bend without breaking; ductile metals will stretch into wires; conductive materials will allow current to flow.
-
The iron nail will gradually form rust, indicating corrosion.
Observation
-
Which metals reacted most and least with water and acid?
-
What visible changes occurred in the displacement reaction?
-
Which materials conducted electricity, and which did not?
STEM Concepts Behind It
Science: Chemical reactivity, displacement, oxidation, and reduction reactions
Technology: Material selection for tools, wires, construction
Engineering: Use of corrosion-resistant metals in infrastructure
Math: Measuring reaction rates, time, and temperature changes
Concept Elaboration
The difference in reactivity arises due to electron configuration metals like sodium have loosely bound outer electrons and react readily, while iron is more stable. The displacement reaction shows metal reactivity order, where a more reactive metal replaces a less reactive one from its compound. Corrosion is a slow chemical change where metals oxidize, especially in the presence of water and oxygen. Properties like malleability and conductivity are linked to metallic bonding, which allows electrons to move freely.
Real-Life Applications
-
Displacement reactions are used in metal extraction and electroplating
-
Reactivity series guides how metals are stored or handled
-
Conductive metals like copper are used in electrical wiring
-
Rust prevention is crucial in bridges, pipelines, and vehicles
-
Malleable metals are shaped into tools, utensils, and machinery parts
Your STEM Challenge
Try creating your own mini corrosion lab: Set up three iron nails one in plain water, one in salty water, and one coated with oil. Predict which rusts fastest and record changes over a week. Design a method to prevent or slow corrosion. Can you invent a new rust-proof coating?
Think Further & Explore More
-
What makes noble metals like gold and platinum so unreactive?
-
How are reactive metals like sodium safely stored and used in real-life?
-
Explore electrolysis: How can we extract pure metals from ores using electricity?
-
Investigate smart metals or shape-memory alloys how do their properties differ?
Let the challenge begin test, observe, and uncover the secrets of metals and non-metals through the power of STEM.
Watch Here – Surface Tension experiment Video
Click Here – Magnetism Experiment For Class 5